We are proud to support world-class lung transplant research and of what we have achieved with your support.
Since inception, we have donated around one million dollars towards research and counting!
What started off as funding research into lung (organ) rejection post transplant has now also translated to better matching at the start of the recipient journey. This means that all organ donors now have a better chance of longer normal life. This is just one way Lungitude is leading the way in supporting world’s best practice in translational research.
The Lungitude Foundation also continues to be a major benefactor of The Alfred’s Lung Transplantation program which remains the premier lung transplant service in Australia, and 5th largest program internationally. Lung transplant activity continues to grow each year, along with ongoing significant improvements in patient survival. The Alfred’s clinical research focus remains on how to better identify, prevent and/or treat several of these key factors: a) AMR ‘antibody mediated rejection’ and b) the link between virus(es) (particularly CMV), infection and development of chronic rejection.e following research.
Of course, research like this could not occur without your ongoing support and donations.
Scroll down to view the Research Fellowships & Projects we fund.
If you are interested in applying for funding, please refer to Grants.
$220,619 for the 2023/24 Financial Year
The Alfred’s Lung Transplant Service have had a very successful and busy year, both from a clinical and research perspective. Clinically, annual lung transplant activity has stabilised, and thankfully the impact of COVID is lessening. They have also had the opportunity of showcasing their research output at important national and international transplant meetings.
Their key findings from the past year include:
- Developing a deeper understanding of how donor and recipient HLA (tissue typing) matching and mis-matching affects long-term lung transplant outcomes. Additionally, advanced technological platforms have also provided new ways of assessing donor and recipient matching, and the results from these new systems are already being utilised to help determine the transplant rejection risk for individual patients- (particularly in the setting of infection and cancer transplant complications)
- There are clues in some of the cells found in lung fluid (BAL) collected in the early weeks after transplant that link to a risk of developing chronic lung rejection (CLAD). Finding these cell signals early after lung transplant may lead to interventions that can prevent development of CLAD.
- They have identified specific donor lung ‘passenger cells’ which are transplanted with the donor lung, which can help transplant recipients control important infections such a cytomegalovirus (CMV) for months after transplant. They continue to look at these donor passenger CMV-specific immune cells to work out exactly how they help may control CMV infection locally in the lungs, which then indirectly protects the lung transplant patient from developing CLAD.
The coming year will see the research at The Translational Lung Transplant Research Hub at Monash University (Alfred Campus) continuing and still firmly focused on reducing the incidence of CLAD post lung transplantation, with the following projects extended and expanding their previous 2 years work through existing and new collaborations.
The Jeff Gittus Lung Transplant Fellowship was announced in 2019, funded generously by Liz Gittus and the Gittus Family. The Fellowship provides one annual fellowship grant of up to $10,000 for a research project of 12-18 months total duration, that focuses specifically on improving lung transplant patient outcomes.
For further information please visit our Grants Section.
The inaugural Jeff Gittus Fellowship, funded by the Gittus Family in memory of Jeff, was awarded to Dr Louise Fuller (Physiotherapist) and Ms Christie Emsley (Dietician) for their project – Body Composition and Muscle Morphology after Lung Transplant.
Update for 2022/2023
Originally, this project was on due to commence in April 2020. Unfortunately, due to the COVID-19 pandemic, all work on new research projects was ceased in late March until further notice. This project will be recommenced in early 2023.
The 2nd Jeff Gittus Fellowship will also be open for applications with more details to come. Future Fellowships will be $20,000 every two years.
Zoe and her family have been supporters of Lungitude since its inception, and we are honoured to contribute to Zoe’s legacy of helping others by establishing a new Zoe Brookes Paediatric Fellowship.
This fellowship is made possible through Lungitude’s fundraising endeavours, including events like our Giving Day. The aim is to sponsor initiatives to aid young paediatric lung transplant recipients, who often face unique challenges due to prolonged illnesses, setting them apart from their peers.
IN COLLABORATION WITH VICTORIAN TRANSPLANTATION AND IMMUNOGENETICS SERVICE (VTIS) TEAM OF STEVEN HIHO AND DR LUCY SULLIVAN.
Steven Hiho and the immunology research team have previously focused on identifying the immunological factors of donors and recipient’s HLA (ie. their tissue typing) to better predict the best match between donor and recipients, and reduce the impact of HLA antibodies (called donor specific antibodies or DSA) that develop in lung transplant recipients not well matched to the donor. It is well known that having DSA contributes to development of chronic lung rejection (CLAD).
To extend this work, the team wish to investigate a previously poorly understood group of ‘non-HLA antibodies’ that some transplant recipients appear to develop, to see if this can help explain further why lung rejection sometimes occurs when there is not the traditionally recognised HLA mismatching between donor and recipients.
IN COLLABORATION WITH DR BRAD GARDINER, ALFRED HEALTH
Optimising immunosuppression to prevent rejection, particularly CLAD, and at the same time minimising the risk of infection remains one of the major challenges after lung transplantation. This project uses the HLA donor-recipient matching data and immune-biomarkers already identified in their research to date, as well using a new blood test (used previously only to assess CMV infection risk) to try and develop a predictive tool to enable individualisation of dosing of immune-suppressing medication that will also see reduced infection risk.
IN COLLABORATION WITH DR DIMITRA ZOTOS, MONASH UNIVERSITY AND RED CROSS LIFEBLOOD.
The researchers have recently identified a new cell marker (biomarker) in patients who developed chronic rejection (CLAD). The laboratory research team plan to use a specifically developed assay to measure this new biomarker in blood samples provided by Red Cross Lifeblood, and compare the results to those found in blood samples already collected over the past 2 years during the current research projects. It is hoped that this new biomarker may help them predict future chronic rejection (CLAD) risk. Additionally, and with financial backing from CSL Ltd, the research team has started a new lung transplant biobank initiative that looks to better understand the causes of primary graft dysfunction.
Hear directly about these exciting research findings at our annual Lungitude Lung Transplantation Research Presentation in October
$135,000 for the 2022/23 Financial Year plus equipment
The Alfred’s Lung Transplant Service continues to be the Australia’s premier lung transplant program, and despite the ongoing impact of COVID-19 on both patients and staff, the clinical and research lab teams have worked hard in very trying circumstances to ensure research project milestones are being met.
Thanks to the generous commitment of significant funding over 3 years by Lungitude Foundation, the setting up of the Translational Lung Transplant Research Hub at Monash University (Alfred Campus) has become a reality with the employment of a fantastic new senior post-doctorial fellow, Dr Sanda Stankovic, in July 2021 and the more recent employment of an excellent laboratory research assistant Ellen Reilly.
Sanda and Ellen have continued the great collaboration the team already had with their other Monash laboratory partners, as well as their clinical lung transplant research team. The following research results being outlined are evidence of what has been achieved by the new team in the last 6-12 months, working on several different aims outlined in the 3 key research projects put forward for support last year:
- Reassessing donor-recipient matching to improve lung transplantation outcomes (Alfred Ethics Project #430-17)
- Harnessing donor immune cells to prevent lung transplantation rejection (Alfred Ethics Project #430-17)
- Harnessing non-conventional T cells for antiviral cellular therapies (Alfred Ethics Project #430-17)
Additionally, Stephen Hiho has made great progress with his PhD this past 12 months, as his report also indicates. He has had to combine working in a very busy tissue typing laboratory during a stressful time over COVID, with continuing his research ‘Assessing new tools for improving donor-recipient matching: (Alfred Ethics Project: 478/19)’ which also addresses some of the aims of Key project #1.
To help reduce the incidence of rejection for lung transplant recipients, the use of immunosuppressive medications are required; however, these same medications make it difficult for the recipient’s body to control infections. One infection that is particularly problematic is a common and often harmless virus, called cytomegalovirus or CMV. About half of all Australians are infected with CMV, but it doesn’t cause major symptoms in healthy people.
However for lung transplant recipients, CMV infection can lead to very harmful complications, including inflammation, pneumonia, rejection (acute and chronic-CLAD) and in severe cases it can result in death. Recipients who experience CMV infection have a higher rate of rejection, and a death rate 6 times higher than those without CMV.
In prior research work, The Alfred researchers have identified a group of immune cells called CMV gamma delta (γδ) T cells that can recognise and control CMV infection. This exciting project focuses in more depth on CMV γδ T cells, as described in the 3 aims below, with the ultimate goal of harnessing this specific cell subset to develop a CMV γδ T cellular therapy(s) and hopefully decrease the impact of CMV infection on lung transplant patient outcomes.
Aim 1: Elucidate the characteristics of CMV γδ T cells in healthy individuals and lung transplant recipients, allowing researchers to determine relevant subsets to be targeted for future cellular therapy.
To address this aim, researchers performed a gene expression on over 700 genes from a sorted population of CMV (NKG2C+) gδT cells from healthy donor blood and compared to non-CMV gδT cells from the same individuals. This allows them to identify key features of CMV-specific gδT cells in a healthy state (Figure 1). This data will then be compared to gδT cells from LTx patients as they continue this work.
Aim 2: Determine the ability of CMV γδ T cells to kill cells infected with CMV. This aim will provide further verification that CMV γδ T cells can control CMV infection and will allow researchers to determine how effective they are compared to conventional cellular therapies.
To undertake this aim, the research team are in the process of setting up a CMV infection experimental system in their laboratory in the next several months. This work has been presented at the Respiratory Department Meeting, Departments of Immunology and Pathology, Monash University, and at the Transplant Research Group meetings@ Alfred Hospital.
Aim 3: Define the mechanisms of how CMV γδ T cells kill cells infected with CMV. This aim will allow researchers to harness these mechanisms for the design of future cellular therapies.
Timeline: 3 years of funding is required for analysis of stored clinical samples collected as part of the Biomarker II Project (Alfred Ethics Project # 430-17) and the generation and completion the of high quality and publishable data to complete the 3 project aims. None of these project aims are contingent on each other, work can commence immediately on most aims concurrently.
The Lungitude Foundation has agreed to fund 50% of the required funding and to assist where possible to help secure the remaining funding alongside The Alfred Lung Transplant Research Team.
Image – CMV

Lung transplantation is the only life-saving procedure for end stage lung diseases but up to 50% of lung transplants develop chronic rejection (Chronic Lung Allograft Dysfunction- CLAD) within the first five years. Although the exact mechanisms underpinning such poor survival are not well understood, the lung is quite different to most other transplanted organs due to the large number of immune cells that belong to the donor that are transplanted with the lung.
Normally, immune cells that live in the lung (“resident immune cells”) regulate lung health and control infections. However, what happens to these resident immune cells upon transplantation is currently unknown. Some of the researchers own data from earlier studies show that the presence of donor-derived resident immune cells long-term in the recipient’s lungs correlates with less CLAD development within first 3 years, implying these may offer protection to the transplanted lung.
Their hypothesis is that the presence of donor-derived lung resident immune cells can provide long-term ‘lung health’, thereby controlling graft rejection, and over time these donor-derived resident immune cells may become lost and not replaced by suitable recipient-derived counterparts.
In this project, researchers will explore the characteristics of lung resident immune cells in transplanted lungs from both healthy patients and those who are developing CLAD. They will assess if these donor-derived resident immune cells offer protection to transplanted lungs and investigate how they are might be changed during development of CLAD, with the ultimate aim of harnessing any potential of these resident immune cells to prevent the development of CLAD post-transplant.
Aim 1: Assess the link between the persistence of donor-derived lymphocytes (resident immune cells) and allograft outcomes. This aim will determine the characteristics and function of donor-derived Immune cells and how they are associated with lung transplant survival.
The donor-derived immune cells (lymphocytes) that are transferred within the allograft appear to be associated with protection from allograft damage. The details of which cell subsets persist and the mechanism of protection they offer is not well understood.
The research teams’ comprehensive study used clinical samples from lung transplant (LTx) recipients such as blood and bronchoalveolar lavage (lung fluid =BAL) to characterize donor-derived lymphocytes and to correlate their proportion with long-term allograft health.
They found that donor-derived lymphocytes are present in a higher proportion in the BAL than in blood post-LTx in all individuals, indicating that the migration out of the donor lung is minimal after transplant. This also suggests that any effects of donor-derived lymphocytes on allograft health are locally mediated.
Furthermore, donor-derived cells persist for longer in the lung suggesting the lung environment is more conducive to their survival. These cells largely consist of T cells and NK cells. When assessing the correlation between the proportion of donor-derived lymphocytes in the lung and CLAD, the researchers observed that LTx recipients with a higher proportion of donor-derived lymphocytes had a lower incidence of subsequent CLAD development, indicating that donor-derived lymphocytes are associated with protection from CLAD.
Further work by the research team will include more patients, especially more CLAD patients, to provide a strong basis for possible diagnostic utility. The researchers highlighted that, interestingly, donor-derived lymphocytes failed to undergo substantial expansion in the lung post-LTx, in contrast to recipient-derived cells, which could explain their loss over time in the recipient (data not shown).
When the researchers explored total cell subset composition between CLAD and CLAD-free “no CLAD” groups (including both donor- and recipient-derived immune cells), they noted that NK cells were significantly increased in the CLAD group between 9 and 18 months post-LTx. This data points to the role of NK cells in the development of lung allograft damage. The team will continue to explore the role of NK cells in CLAD in more detail.
Aim 2: Assess the contribution of acute lung injury to lung “resident” immune cells. This aim will determine if acute rejection or infection can change lung “resident” immune cells.
The researchers shared that cells termed ‘tissue-resident’, are located directly within the lung epithelium (lung lining), along the lung airways. These cells have been shown to play a key role in the local anti-viral immunity as a first-line defence against pathogens and have been identified to express marker CD103 on the cell surface.
As cytomegalovirus (CMV) is a pathogen that has been associated with worse lung transplant outcomes, the researchers aimed to explore if donor-derived cells in this location are associated with protection from CMV reactivation in the lung.
Using BAL samples, they discovered that the proportion of tissue resident donor-derived cells was higher in the LTx recipients without CMV reactivation.
This indicated to the researchers that either these cells are more capable of expanding after LTx, or that it was their high proportion in the first place that offered strong anti-viral (anti-CMV) protection.
The researchers also noted a higher proportion of HLA-matching between donor and recipient in LTx individuals who did not have CMV reactivation in the lung. This offered a hypothesis that it is the recognition of the ‘similar’ HLA in the recipient that may allow donor-derived cells to protect from CMV reactivation.
Future work will focus on this observation and the role HLA matching may have in the protection from virus reactivation.
Aim 3: Assess the contribution of chronic lung injury to lung “resident” immune cells. This aim will evaluate whether lung “resident” immune cells differ between healthy, non-transplanted lungs and the failed transplanted lung.
Timeline: 3 years of funding is required for analysis of stored clinical samples collected as part of the Biomarker II Project (Alfred Ethics Project # 430-17) and the generation and completion the of high quality and publishable data to complete the 3 project aims. None of these project aims are contingent on each other, work can commence immediately on most aims concurrently.
The Lungitude Foundation has agreed to fund 50% of the required funding and to assist where possible to help secure the remaining funding alongside The Alfred Lung Transplant Research Team.
Image credit – Dr Sanda Stankovic PhD

Lung transplantation transforms the lives of patients with end-stage lung failure, but long-term survival is limited by chronic rejection, called chronic lung allograft dysfunction (CLAD). An increased understanding of the mechanisms of CLAD is urgently required to develop strategies to minimise and treat CLAD to improve recipient survival. A major barrier to achieving longer term post-transplant survival is difficulty matching specialised proteins called human leukocyte antigens (HLA) between donors and recipients.
Typically, 2 proteins called HLA-A and HLA-B are matched as close as possible between donors and recipients, while a third variant, HLA-C, has been largely ignored in this process. Recently the researchers have been exploring some exciting (but as yet unpublished) preliminary data which shows that mismatching of donor and recipient HLA-C profoundly impacts on the development of CLAD, however the mechanism why this is so is unclear.
By looking specifically at HLA-C they are undertaking a more novel approach to donor-recipient matching and hoping to reduce the incidence of CLAD. This may ultimately contribute to an “HLA-C selection” algorithm for all solid organ transplant recipients.
Aim 1: Define the contribution of both donor and recipient HLA-C to the development of CLAD. This aim will determine how donor HLA-C can change the proportion and characteristics of recipient immune cells and determine the extent to which they are predictive of CLAD.
Aim 2: Define the impact of donor HLA-C on immune cell function. This aim will determine the extent to which donor HLA-C impacts on the function of recipient immune cell subsets.
Aim 3: Define the impact of donor HLA-C on antibody-mediated rejection. This aim will determine whether mismatched HLA-C can lead to the formation of antibodies to HLA-C in the recipient. Researchers will also assess whether the formation of these antibodies can lead to the development of CLAD.
Timeline: 3 years of funding is required for analysis of stored clinical samples collected as part of the Biomarker II Project (Alfred Ethics Project # 430-17) and the generation and completion the of high quality, and publishable data to complete each of the 3 project aims. None of these project aims are contingent on each other, work can commence immediately on most aims concurrently.
The Lungitude Foundation has agreed to fund 50% of the required funding and to assist where possible to help secure the remaining funding alongside The Alfred Lung Transplant Research Team.
Image – Steven Hiho

Lungitude has committed to the funding a PhD candidate, Steven Hiho, as he continues to work on projects to improve donor- recipient matching.
The last few years has seen the creation of several new computer programs to assess ‘compatibility’ between a lung transplant recipient and a potential donor. These programs use the differences between donor and recipient proteins to give a ‘score’ of compatibility between any particular pair.
Developing better tools for donor- recipient matching may be a key to preventing the development of CLAD, with the added aims of improving and prolonging life post lung transplantation.
Recent Progress
Expanding on the results of early project work, Steven has continued to work with the VITS/Red Cross tissue typing team to try and work on the best way to use HLA epitopes and epMM scoring clinically, plus better HLA screening of donors, to be able to select more compatible recipients for each potential donor. He is also extending his PhD research to look at other (non-HLA) immunological markers which could also potentially be used in the pre-transplant compatibility assessment of lung donors.
The overall aim of Steven’s PhD was to identify immunological factors, which can be used to match donor and recipients to provide better outcomes. So far, the research has shown that the use of HLA compatibility, specifically B-cell epitopes, provide a way of evaluating the risk of antibody formation and rejection post- transplant, and that limiting epitope mismatching between recipient and donor increases long term survival following a lung transplant.
The research investigated all ‘epitope’ algorithm’s available, and determined which algorithms and epitope mismatch levels; best predicts long-term survival and reduces risk of rejection. The goal is to incorporate this into the pre- transplant assessment through RedCross Lifeblood once the current manuscript is published, “Comparison of HLA immunological risk stratification methods in lung transplantation” (Late 2022).
The second aim of Steven’s PhD was to determine which method most accurately defines the pre-transplant antibodies, which are of concern in a recipient-donor pair. This work investigated both the flow cytometry and virtual assessment methods to evaluate whether the use of a virtual assessment can provide the pretransplant antibody assessment. The research demonstrated that a virtual assessment is a faster and more accurate way to define these dangerous antibodies. This significant work has provided, not only the lung transplant teams, but other Australian based transplant units the ability to move forward with the use of a virtual assessment of transplant risk.
Future directions of this PhD will include investigating the role of nonHLA antibodies in lung transplantation. From the work with the flow and virtual crossmatching, the research identified that non-HLA antibodies interact with cells on these assays and the wish is to determine any clinical impact this has on recipients, and whether these non-HLA antibodies need to be incorporated into the pre-transplant assessment.
Image – Steven Hiho
Publications
The clinical utility and thresholds of virtual and Halifaster flow crossmatches in lung transplantation. Hiho SJ, Levvey B, Carroll R, Nicolson I, Mihaljcic M, Diviney MB, Snell GI, Sullivan LC, Westall GP. HLA. 2022 Mar 27.
Determining clinical thresholds for donor HLA eplet compatibility to predict best outcomes following lung transplantation. (Accepted Manuscript Transplantation Direct 2022) Hiho. Steven J, Walton. Duncan. C, Paraskeva. Miranda, Levvey. Bronwyn. J, Diviney. Mary. B, Snell. Gregory. I, Sullivan. Lucy. C, Westall. Glen. P.
Major technological advances will enhance Australian donor- recipient matching and improve transplant outcomes (under review IMJ) Steven Hiho, Bronwyn Levvey, Rhonda Holdsworth, Lucy Sullivan, Glen Westall, Greg Snell
Comparison of HLA immunological risk stratification methods in lung transplantation (Manuscript in progress) Target journal AJT Late 2022 Impact of reporting HLA alleles from Real-Time PCR on deceased donor DSA assessments and conformance with high resolution alleles. (Manuscript in progress) Target Journal Human Immunology Aug 2022 Steven Hiho, Sue Bowman, Fiona Hudson, Lucy Sullivan, Robert Carroll, Mary Diviney
Awards and Grants
2022 TSANZ President Prize Finalist 2022
TSANZ Early Career Researcher Award (Clinical science)
2019 Transplant Research Advisory Committee (TRAC) Grant ($53,500)
Presentations and Conferences
2022 TTS (Oral) Comparison of HLA compatibility algorithms to predict longterm survival and CLAD following lung transplantation
2022 TSANZ (President Prize Oral) The clinical utility and thresholds of virtual and Halifaster flow crossmatches in lung transplantation
2022 TSANZ (Oral) Role of non-HLA in Lung transplantation
2020 TSANZ (Poster) Use of HLA epitopes in a virtual crossmatch to better assess lung transplant compatibility
2020 ISHLT (Virtual Oral) HLA Epitope Mismatch Load (epMM) Allows Classification of Immunological Risk and Correlates with Patient Survival Following Lung Transplantation (LTx)
2020 ISHLT (Virtual Oral) Can Avoiding So Called HLA High Risk Epitope Mismatches (REM) Improve Lung
$160,000 for the 2021/22 Financial Year

The Jeff Gittus Lung Transplant Fellowship was announced in 2019, funded generously by Liz Gittus and the Gittus Family. The Fellowship provides one annual fellowship grant of up to $10,000 for a research project of 12-18 months total duration, that focuses specifically on improving lung transplant patient outcomes.
For further information please visit our Grants Section.
The inaugural Jeff Gittus Fellowship, funded by the Gittus Family in memory of Jeff, was awarded to Dr Louise Fuller (Physiotherapist) and Ms Christie Emsley (Dietician) for their project – Body Composition and Muscle Morphology after Lung Transplant.
Update for 2021/2022
Originally, this project was on due to commence in April 2020. Unfortunately, due to the COVID-19 pandemic, all work on new research projects was ceased in late March until further notice. This project will be recommenced in 2022.
The 2nd Jeff Gittus Fellowship will also be open for applications with more details to come. It is expected that going forward the Fellowship will be $20,000 every two years.

Immunosuppressive medications are required to prevent rejection following lung transplantation; however, these same medications make it difficult for the body to control infections. One infection that is particularly problematic following lung transplantation is a very common and normally harmless virus, called cytomegalovirus or CMV. About half of all Australians are infected with CMV, but the virus survives without causing major symptoms in healthy people.
However, CMV infection in lung transplant recipients can lead to very harmful complications, including inflammation, pneumonia, rejection (acute and chronic-CLAD) and in severe cases where treatments fail, it can result in death. Indeed, lung transplant patients who experience CMV infection have a higher rate of rejection, and a death rate 6 times higher than those without CMV. Therefore, the control of CMV infection is critical for ensuring positive outcomes following lung transplantation.
Current therapies to treat CMV have significant side effects including nausea, diarrhea, vomiting and can result in the loss of kidney function. Additionally, some patients can develop resistance to the current therapies used to prevent and treat CVM infection. Hence, there is an urgent unmet need to provide novel treatments for CMV infection.
In prior research work, The Alfred researchers have identified a group of immune cells called CMV gamma delta (γδ) T cells that can recognise and control CMV infection. This exciting project will focus in more depth on CMV γδ T cells, as described in the 3 aims below, with the ultimate goal of harnessing this specific cell subset to develop a CMV γδ T cellular therapy(s) and hopefully decrease the impact of CMV infection on lung transplant patient outcomes.
Aim 1: Elucidate the characteristics of CMV γδ T cells in healthy individuals and lung transplant recipients. This aim will allow researchers to determine relevant subsets to be targeted for future cellular therapy.
Aim 2: Determine the ability of CMV γδ T cells to kill cells infected with CMV. This aim will provide further verification that CMV γδ T cells can control CMV infection and will allow researchers to determine how effective they are compared to conventional cellular therapies.
Aim 3: Define the mechanisms of how CMV γδ T cells kill cells infected with CMV. This aim will allow researchers to harness these mechanisms for the design of future cellular therapies.
Timeline: 3 years of funding is required for analysis of stored clinical samples collected as part of the Biomarker II Project (Alfred Ethics Project # 430-17) and the generation and completion the of high quality and publishable data to complete the 3 project aims. None of these project aims are contingent on each other, work can commence immediately on most aims concurrently.
The Lungitude Foundation has agreed to fund 50% of the required funding and to assist where possible to help secure the remaining funding alongside The Alfred Lung Transplant Research Team.
Image – Professor Glen Westall

Lung transplantation is the only life-saving procedure for end stage lung diseases but up to 50% of lung transplants develop chronic rejection (Chronic Lung Allograft Dysfunction- CLAD) within the first five years. Although the exact mechanisms underpinning such poor survival are not well understood, the lung is quite different to most other transplanted organs due to the large number of immune cells that belong to the donor that are transplanted with the lung.
Normally, immune cells that live in the lung (“resident immune cells”) regulate lung health and control infections. However, what happens to these resident immune cells upon transplantation is currently unknown. Some of the researchers own data from earlier studies show that the presence of donor-derived resident immune cells long-term in the recipient’s lungs correlates with less CLAD development within first 3 years, implying these may offer protection to the transplanted lung.
Their hypothesis is that the presence of donor-derived lung resident immune cells can provide long-term ‘lung health’, thereby controlling graft rejection, and over time these donor-derived resident immune cells may become lost and not replaced by suitable recipient-derived counterparts.
In this project, researchers will explore the characteristics of lung resident immune cells in transplanted lungs from both healthy patients and those who are developing CLAD. They will assess if these donor-derived resident immune cells offer protection to transplanted lungs and investigate how they are might be changed during development of CLAD, with the ultimate aim of harnessing any potential of these resident immune cells to prevent the development of CLAD post-transplant.
Aim 1: Assess the link between the persistence of donor-derived lymphocytes (resident immune cells) and allograft outcomes. This aim will determine the characteristics and function of donor-derived Immune cells and how they are associated with lung transplant survival.
Aim 2: Assess the contribution of acute lung injury to lung “resident” immune cells. This aim will determine if acute rejection or infection can change lung “resident” immune cells.
Aim 3: Assess the contribution of chronic lung injury to lung “resident” immune cells. This aim will evaluate whether lung “resident” immune cells differ between healthy, non-transplanted lungs and the failed transplanted lung.
Timeline: 3 years of funding is required for analysis of stored clinical samples collected as part of the Biomarker II Project (Alfred Ethics Project # 430-17) and the generation and completion the of high quality and publishable data to complete the 3 project aims. None of these project aims are contingent on each other, work can commence immediately on most aims concurrently.
The Lungitude Foundation has agreed to fund 50% of the required funding and to assist where possible to help secure the remaining funding alongside The Alfred Lung Transplant Research Team.
Image credit – Dr Sanda Stankovic PhD

Lung transplantation transforms the lives of patients with end-stage lung failure, but long-term survival is limited by chronic rejection, called chronic lung allograft dysfunction (CLAD). An increased understanding of the mechanisms of CLAD is urgently required to develop strategies to minimise and treat CLAD to improve recipient survival. A major barrier to achieving longer term post-transplant survival is difficulty matching specialised proteins called human leukocyte antigens (HLA) between donors and recipients.
Typically, 2 proteins called HLA-A and HLA-B are matched as close as possible between donors and recipients, while a third variant, HLA-C, has been largely ignored in this process. Recently the researchers have been exploring some exciting (but as yet unpublished) preliminary data which shows that mismatching of donor and recipient HLA-C profoundly impacts on the development of CLAD, however the mechanism why this is so is unclear.
By looking specifically at HLA-C they are undertaking a more novel approach to donor-recipient matching and hoping to reduce the incidence of CLAD. This may ultimately contribute to an “HLA-C selection” algorithm for all solid organ transplant recipients.
Aim 1: Define the contribution of both donor and recipient HLA-C to the development of CLAD. This aim will determine how donor HLA-C can change the proportion and characteristics of recipient immune cells and determine the extent to which they are predictive of CLAD.
Aim 2: Define the impact of donor HLA-C on immune cell function. This aim will determine the extent to which donor HLA-C impacts on the function of recipient immune cell subsets.
Aim 3: Define the impact of donor HLA-C on antibody-mediated rejection. This aim will determine whether mismatched HLA-C can lead to the formation of antibodies to HLA-C in the recipient. Researchers will also assess whether the formation of these antibodies can lead to the development of CLAD.
Timeline: 3 years of funding is required for analysis of stored clinical samples collected as part of the Biomarker II Project (Alfred Ethics Project # 430-17) and the generation and completion the of high quality, and publishable data to complete each of the 3 project aims. None of these project aims are contingent on each other, work can commence immediately on most aims concurrently.
The Lungitude Foundation has agreed to fund 50% of the required funding and to assist where possible to help secure the remaining funding alongside The Alfred Lung Transplant Research Team.
Image – Steven Hiho

Lungitude has committed to the funding a PhD candidate, Steven Hiho, as he continues to work on projects to improve donor- recipient matching.
The last few years has seen the creation of several new computer programs to assess ‘compatibility’ between a lung transplant recipient and a potential donor. These programs use the differences between donor and recipient proteins to give a ‘score’ of compatibility between any particular pair.
Developing better tools for donor- recipient matching may be a key to preventing the development of CLAD, with the added aims of improving and prolonging life post lung transplantation.
Recent Progress
Expanding on the results of early project work, Steven has continued to work with the VITS/Red Cross tissue typing team to try and work on the best way to use HLA epitopes and epMM scoring clinically, plus better HLA screening of donors, to be able to select more compatible recipients for each potential donor. He is also extending his PhD research to look at other (non-HLA) immunological markers which could also potentially be used in the pre-transplant compatibility assessment of lung donors.
Image – Steven Hiho

Lung transplantation (LTx) is an established therapeutic option to improved survival and quality of life for selected patients with advanced severe lung disease. However, the survival after LTx is a median of 6.5 years world-wide, and remains inferior to survival demonstrated in other solid organ transplant groups. Chronic Lung Allograft Dysfunction (CLAD) is regarded as the leading determinant of poor patient survival and the most common manifestation of CLAD is an obstructive phenotype, also commonly known as bronchiolitis obliterans syndrome (BOS) characterised by airflow limitation. There is also another common phenotype of CLAD which is characterised by decreased lung volumes and fibrosis of the lung tissue- this is called restrictive allograft syndrome (RAS) which has a particularly grim prognosis. The identification of predictive factors associated with survival and development of CLAD is imperative to improve outcomes for lung transplant recipients.
The current standard of care utilises spirometry, a routine measurement of lung function, to monitor graft function longitudinally and detect development of rejection after lung transplantation. However, there are significant clinical limitations to spirometric monitoring, as it is relatively insensitive to early and subtle changes in lung function in transplanted lungs. Furthermore, spirometry is not sensitive enough to detect changes in function within the small airways or the alveolar-capillary interface (lung air sacs).
The Forced Oscillometry technique (FOT) is a new ‘simpler’ pulmonary function test that uses ‘normal breathing’ with-out requiring a ‘forced effort’ like spirometry, and uses pressure waves to measure respiratory mechanics and function in the small airways where the injury of CLAD more commonly occurs. FOT testing adds approximately 5 minutes extra to the standard lung function testing process. Studies in different types of lung disease, such as asthma and chronic obstructive lung disease, have shown that FOT can detect lung diseases changes earlier than conventional pulmonary function tests such as spirometry.
Recent Progress in 2020 & 2021
Despite the impact of COVID in 2020 and the inability for patients to physically attend the Alfred to have lung function testing, the ‘FOT in Lung Transplantation’ project was able to eventually commence, with the first patient being consented, enrolled and tested at The Alfred in Aug 2020. Brigitte Borg (Head of the Physiology/Lung Function Dept) and Kris Nilsen (Senior scientist- Physiology/Lung Function Dept- funded by Lungitude Foundation 2 days per week) set up a streamlined process within the lung function department to consent and enrol patients into 2 FOT studies. Dr Jai Vazirani (LTx Consultant) and Dr Tom Crowhurst (LTx Fellow) joined the research team and assisted with consenting and enrolment of patients when they attend the transplant clinic for review.
Currently the focus has been on 2 studies being done evaluating the benefit of FOT, and already researchers are obtaining some really important information which has been presented recently at national and international conferences:
Study # 1 update: This is a cross-sectional study where FOT measure measurements are done at one time point, at the same time enrolled study patients have their other routine lung function tests done, to determine if FOT is a better detector of early CLAD versus standard spirometry. This study currently has 57 patients enrolled to date at Alfred and approximately 200 at St Vincent’s Hospital, Sydney. Results from an early analysis in 2 selected cohorts of patients from this study are quite promising, and 2 abstracts submitted to the ISHLT Virtual Meeting (held 24-28 April,2021) were selected to be presented; one as highly selected oral presentation1 and one as a poster presentation2 .
Study # 2 update: This is a longitudinal study which follows patients early from lung transplant and measures FOT along with other routine lung function measures at their first post-transplant lung function test, then monthly during the first 12 months, and 3 monthly thereafter. This study will determine the ability of using FOT testing post LTx to detect early CLAD, and also characterise the different physiological and mechanical changes that occur in the chest wall with LTx. Currently 44 patients at the Alfred have been enrolled and are participating in this longitudinal study, and 37 from St Vincents, Sydney. Enrolments will continue at both hospitals during 2021 and analysis of data will be done as cohorts of the patients reach 12 months post-LTx.
Study # 3 update: This study has only just commenced as there had been a delay in 2020 due to COVID in re- starting the pre-Tx assessment lung function testing. Enrolments for this study are only being done at The Alfred at present.
Presentations on research outcomes related to this project to date:
Oral Presentation @ ISHLT, April 2021- Characterisation of Baseline and Chronic Lung Allograft Dysfunction by Airway Oscillometry: Results of a Multi-centre Cross-Sectional Study. First author and Presenter-David Darley (St Vincent’s, Sydney), J Sim, K Nilsen, R Shirol, B Borg, J Vazirani, B Levvey, G Snell, M Plitt, K Tonga
Poster @ ISHLT, April 2021: Novel measurements of respiratory system mechanics demonstrate important features of donor-recipient lung volume matching that may link to subsequent chronic lung allograft dysfunction. First author and Presenter- Kris Nilsen (Alfred), D Darley, B Levvey, B Borg, J Vazirani, J Sim, R Shirol, M Plitt, G Snell.
Oral presentation at TSANZSRS/ANZSRS Conference, 30 April 21: Transplant donor-recipient lung-volume matching alters novel measurements of respiratory mechanics. First author and Presenter- *Kris Nilsen (Alfred), D Darley, B Levvey, B Borg, J Vazirani, J Sim, R Shirol, M Plitt, G Snell.
* Kris Nilsen won the ANZSRS Robert Jensen Excellence in Respiratory Measurement Prize for the best presentation at the ANZSRS conference in 2021.
Funding for 2021 & 2022
This project is already showing some excellent and early promising results, as demonstrated by the research abstracts that have been accepted following peer review for presentation at the recent ISHLT and TSANZ SRS conferences.
Thus, researchers have sought and been granted funding support from Lungitude for the 2021-2022 financial year to help fund the 2 day/week salary for Dr Kris Nilsen- senior physiologist/ scientist and a lead researcher for this project.
We can already see the very positive benefits of this project and research direction. The researchers are very excited by this project, as they believe that the simple, non-invasive lung function test FOT has real potential to pick up much earlier changes in lung function related to CLAD, allowing for earlier intervention and hopefully will lead to improvement in overall LTx clinical outcomes.
Image credit – Tremoflo
Abnormal one-year post-lung transplant spirometry is a significant predictor of increased mortality and chronic lung allograft dysfunction. Paraskeva MA, Borg BM, Paul E, Fuller J, Westall GP, Snell GI.J Heart Lung Transplant. 2021. Online ahead of print
Outcomes Following ATG Therapy for Chronic Lung Allograft Dysfunction. Kotecha S, Paul E, Ivulich S, Fuller J, Paraskeva M, Levvey B, Snell G, Westall G.Transplant Direct. 2021 Mar 16;7(4):e681.
Evaluation of Quantiferon®-Monitor as a biomarker of immunosuppression and predictor of infection in lung transplant recipients. Gardiner BJ, Lee SJ, Cristiano Y, Levvey BJ, Sullivan LC, Snell GI, Peleg AY, Westall GP.Transpl Infect Dis. 2021 Jun;23(3):e13550.
Scedosporium apiospermum and Lomentospora prolificans in lung transplant patients – A single center experience over 24 years. Vazirani J, Westall GP, Snell GI, Morrissey CO.Transpl Infect Dis. 2021 Jun;23(3):e13546.
Outcomes Following Extracorporeal Photopheresis for Chronic Lung Allograft Dysfunction Following Lung Transplantation: A Single-Center Experience. Vazirani J, Routledge D, Snell GI, Watson D, Paraskeva M, Westall GP, Harrison SJ. Transplant Proc. 2021 Jan- Feb;53(1):296-302.
Cytomegalovirus replication is associated with enrichment of distinct γδT cell subsets following lung transplantation: A novel therapeutic approach? Stankovic S, Davey MS, Shaw EM, von Borstel A, Cristiano Y, Levvey BJ, Rossjohn J, Westall GP, Snell GI, Brooks AG, Sullivan LC.J Heart Lung Transplant. 2020 Aug 26;39(11):1300-12.
Molecular T-cell–mediated rejection in transbronchial and mucosal lung transplant biopsies is associated with future risk of graft loss. Halloran K, Parkes MD, Timofte I, Snell G, Westall G, Havlin J, Lischke R, Hachem R, Kreisel D, Levine D, Kubisa B, Piotrowska M, Juvet S, Keshavjee S, Jaksch P, Klepetko W, Hirji A, Weinkauf J, Halloran PF.J Heart Lung Transplant. 2020 Dec;39(12):1327-1337.
Prolonged survival after lung transplantation in the absence of conventional immunosuppression. Vazirani J, Snell GI, Westall GP.J Heart Lung Transplant. 2020 Oct;39(10):1159-1162.
Cost-effectiveness of transplanting lungs and kidneys from donors with potential hepatitis C exposure or infection. Scott N, Snell G, Westall G, Pilcher D, Raggatt M, Walker RG, Hellard M, Peleg AY, Doyle J.Sci Rep. 2020 Jan 29;1 0(1):1459.
Atrial Flutter and Fibrillation Following Lung Transplantation: Incidence, Associations and a Suggested Therapeutic Algorithm. Barnes H, Gurry G, McGiffin D, Westall G, Levin K, Paraskeva M, Whitford H, Williams T, Snell G.Heart Lung Circ. 2020 Oct;29(10):1484-1492.
Does continuation of antifibrotics before lung transplantation influence post-transplant outcomes in patients with idiopathic pulmonary fibrosis? Zhu MZL, Huang JY, Liu DH, Snell GI.Interact Cardiovasc Thorac Surg. 2021 Aug 28
An Audit of Lung Donor Pool: Optimal Current Donation Strategies and the Potential of Novel Time-Extended Donation After Circulatory Death Donation. Okahara S, Levvey B, McDonald M, D’Costa R, Opdam H, Pilcher DV, Snell GI.Heart Lung Circ. 2021.
An Intention-to-treat View of Lung Transplantation for Interstitial Lung Disease: Successful Strategies to Minimize Waiting List and Posttransplant Mortality. Zhu MZL, Levvey BJ, McGiffin DC, Snell GI.Transplantation. 2021 Feb 5.
Improving the predictability of time to death in controlled donation after circulatory death lung donors. Okahara S, Snell GI, McDonald M, D’Costa R, Opdam H, Pilcher DV, Levvey B.Transpl Int. 2021 May;34(5):906-915.
A Retrospective Review of Declined Lung Donors: Estimating the Potential of Ex Vivo Lung Perfusion. Okahara S, Levvey B, McDonald M, D’Costa R, Opdam H, Pilcher DV, Snell GI.Ann Thorac Surg. 2021 Aug;112(2):443-449.
$100,000 for the 2020/21 Financial Year

The Jeff Gittus Lung Transplant Fellowship was announced in 2019, funded generously by Liz Gittus and the Gittus Family. The Fellowship provides one annual fellowship grant of up to $10,000 for a research project of 12-18 months total duration, that focuses specifically on improving lung transplant patient outcomes.
For further information please visit our Grants Section.
The inaugural Jeff Gittus Fellowship, funded by the Gittus Family in memory of Jeff, was awarded to Dr Louise Fuller (Physiotherapist) and Ms Christie Emsley (Dietician) for their project – Body Composition and Muscle Morphology after Lung Transplant.
Update for 2020/2021
Originally, this project was on due to commence in April 2020. Unfortunately, due to the COVID-19 pandemic, all work on new research projects was ceased in late March until further notice.

Lung transplantation transforms the lives of patients with end-stage lung failure. However, long-term survival is limited by the development of chronic rejection, defined as chronic lung allograft dysfunction (CLAD). Indeed, the median survival following lung transplantation is only 7 years, largely due to CLAD. However, the underlying causes of CLAD and why some recipients are more prone to develop it than others remain unclear. Therefore, an increased understanding of the mechanisms that drive CLAD is urgently required to improve lung transplant recipient survival.
This project aimed to investigate a mechanism of CLAD called “Antibody Mediated Rejection” (AbMR). Our immune system is designed to recognise “foreign” invaders such as bacteria and viruses and produce antibodies to fight them off. However, the transplanted lung is also seen as “foreign” to the recipient’s immune system, and antibodies known as donor specific antibodies (DSA) can be produced which attack the transplanted donor lung.
Defeating Transplant Rejection: Antibodies and Strategies to Control Them aims to identify and understand the production of DSAs and identify the mechanisms that lead to damage of the transplanted lung. The funding provided by the Lungitude Foundation/ Alfred Foundation Matched Funding Grant enabled the recruitment of a senior post-doctorate researcher, Dr Lucy Sullivan, to oversee the conduct of this very important project. The significant findings of this research over the past 4 years are highlighted below.
- Better donor-recipient matching
In collaboration with the Australian Red Cross Lifeblood, researchers aimed to determine if computer programs could be used to predict better donor-recipient matches. Initially, their work showed that using a program called “HLA Matchmaker” could predict which recipients would develop CLAD based on “structural similarity”. This research was published in the prestigious journal, American Journal of Transplantation. Moreover, in a small cohort of recipients they showed that better matching also reduced the appearance of DSA. This work was published in the journal Transplant Immunology.
PhD student, Mr Steven Hiho, who is based at the Australian Red Cross LifeBlood, has compared different methods to predict donor-recipient compatibility. He has discovered that the best outcomes following lung transplantation can be achieved by combining a special form of matching called “eplet” matching and avoiding pre-transplant DSA. This data was selected for oral presentation at the International Society for Heart and Lung Transplantation (ISHLT) meeting in Montreal, April 2020. This was a significant achievement as this is the premiere meeting for lung transplantation and very few abstracts are invited to present as an oral presentation. Unfortunately, this conference was cancelled due to the COVID-19 pandemic. Mr Hiho has also used the “HLA Matchmaker” program to define a “cut-off score”. Donor-recipient matches below this score were associated with less CLAD and overall improved survival. This work is important as these scores can be used to guide clinical decisions in donor-recipient matching to prevent antibody-mediated rejection. This work will soon be submitted to the journal Transplantation.
Another aspect of Mr Hiho’s project is to compare donor/recipient matching techniques and relate this to lung transplant outcomes. It is hoped that this research will lead to better methods of donor selection being adopted on a national level.
- Identifying the immune cells involved in AbMR
Identifying and understanding the immune mechanisms that control antibody production and mediate tissue destruction will help identify patients for targeted therapies to prevent graft rejection.
In collaboration with researchers at Monash University, researchers are conducting a project to identify the specific immune cells that produce DSA. They showed that the number of “B cells” is significantly increased in the blood of recipients that have damaging antibodies. They are now using precise reagents on a larger cohort to further elucidate the characteristics of the B cells, and to track their appearance.
Researchers also aimed to identify the immune cells that cause damage to the lung following the production of DSA. They showed that group of white blood cells called “natural killer” or NK cells will kill other cells when antibodies are present. They also showed that not all NK cells are equal and certain groups of NK are more likely to kill other cells when DSA are present. This work was also selected for oral presentation at the cancelled 2020 ISHLT meeting.
- Identifying a role for novel molecules in AbMR and CLAD
In some exciting new research, researchers have been investigating the potential role of novel molecules in the development of CLAD. Normally, donors and recipients are “matched” based on similarities between their proteins called “human leukocyte antigens” or HLA. HLA comes in a variety of forms, but matching is only done by comparing 3 types of HLA, called HLA-A, HLA-B and HLA-DR. Their research has uncovered that matching between another molecule called HLA-C can also have a profound impact on the development of CLAD. As HLA-C has been previously ignored in the matching process, this research potentially represents a paradigm shift in assessing donor-recipient HLA matching. Their future work in this area is aimed at designing ways in which HLA-C can be incorporated into matching programs, thereby improving selection of donor/recipient pairs, preventing antibody-mediated rejection and prolonging the life of the transplanted lung.
Researchers have also commenced a project identifying a role for “unconventional” antibodies to the development of AbMR. They found that following lung transplant, antibodies can develop to harmless proteins that are already part of the body, such as proteins that make up the building blocks of the tissue within the lung. The researchers will continue to pursue the role of these antibodies in the development of AbMR.
- Determining the link between viral infection and AbMR
The researchers have also investigated possible links between infection with a virus called cytomegalovirus (CMV) and antibody-mediated rejection. They have previously observed that CMV replication was associated with the development of CLAD, however the link between two was not well understood. In exciting research, they discovered a new subset of immune cells that can potentially control CMV infection following lung transplantation. This work was accepted for publication in the highest impact transplantation journal, The Journal of Heart and Lung Transplantation. This work was also selected for oral presentation at the cancelled 2020 ISHLT meeting. Their new work is aimed at harnessing these immune cells to prevent CMV infections, thereby also limiting AbMR.
- The role of the donor’s immune system in AbMR
The researchers now believe that the antibodies that are produced by the donor’s immune cells can also contribute to antibodies present in recipients. Their findings were accepted for publication in the journal, American Journal of Transplantation. The now have a Masters of Biomedical Science student, Mr Kane Parsons, on this project. His early findings suggest that immune cells from the donor can survive for a long time in the recipient, up to several months in some cases. However, the number and type of the donor’s immune cells is extremely variable between different recipients. Intriguingly, early data suggests that persistence of donor-derived immune cells protects the recipient from CLAD. This data was recently presented at the Transplant Society of Australia and New Zealand Annual Scientific Meeting (March 2021). They will continue in this area of research as it has the potential to cause a paradigm shift in the way we think about antibodies that can cause damage to a transplanted lung.
Research Outputs
Overall, the researchers have made significant progress on the project “Defeating Lung Transplant Rejection: Antibodies and strategies to control them”. These research findings will translate to fundamental shifts in clinical approaches to patient care and will improve outcomes for future lung transplant recipients. Research outputs are listed below.
Peer-reviewed manuscripts:
- Stankovic S., M. S. Davey, E. M. Shaw, A. von Borstel, Y. Cristiano, B. J. Levvey, J. Rossjohn, G. P. Westall, G. I. Snell, A. G. Brooks and L. C. Sullivan (2020). “Cytomegalovirus replication is associated with enrichment of distinct γδ T cell subsets following lung transplantation: A novel therapeutic approach?” J Heart Lung Transplant. 39:1300-1312
- Snell, G.I., S. Hiho, B. J. Levvey, L.C. Sullivan and G.P. Westall (2019). “Consequences of donor-derived passengers (pathogens, cells, biological molecules and proteins) on clinical outcomes”. J Heart Lung Transplant. 38: 902-906
- Sullivan, L.C., E. M. Shaw, S. Stankovic, G. I. Snell, A. G. Brooks and G. P. Westall (2019). “The complex existence of γδ T cells following transplantation: the good, the bad and the simply confusing”. Invited Review. Clin Transl Immunology 8:e1078
- Harpur, C. M., S. Stankovic, A. Kanagarajah, J. M. L Widjaja, B.J . Levvey, Y. Cristiano, G. I Snell, A. G. Brooks, G. P Westall and L.C. Sullivan (2019). “Enrichment of cytomegalovirus-induced NKG2C+ Natural Killer cells in the lung allograft”. Transplantation. 103:1689-1699.
- Kummrow, M. S. Hiho, F. Hudson, L. Cantwell, W. Mulley, L. D’Orsogna, A. Testro, J. Pavlovic, P. MacDonald, L.C. Sullivan, G. I. Snell and G. P. Westall (2019). “Transfer of donor anti-HLA antibody expression to multiple transplant recipients- a potential variant of the Passenger Lymphocyte Syndrome?” Am J Transplant 19(5):1577-1581.
- Walton, D. C., L. Cantwell, S. Hiho, J. Ta, S. Wright, L. C. Sullivan, G.I. Snell and G.P. Westall (2018). “HLA class II Eplet mismatch predicts De Novo DSA formation post lung transplant”. Transpl Immunol. 51:73-75.
Invited commentaries:
Sullivan, L.C., E. M. Shaw and G. P. Westall (2018). “γδ T Cells in Transplantation: Friend and Foe”. Transplantation. 102:1970-1971.
Book chapters:
Westall, G. P. and L. C. Sullivan (2018). “Antibody Mediated Rejection: Are We There Yet?” Essentials in Lung Transplantation. A.R. Glanville, Springer: 79-86.
Conference Presentations:
2021 Transplant Society of Australia and New Zealand Annual Scientific Meeting (2 presentations)
2020 International Society for Heart and Lung Transplantation (3 presentations, abstracts accepted but conference cancelled)
2020 Transplant Society of Australia and New Zealand Annual Scientific Meeting (3 presentations, abstracts accepted but conference cancelled)
2019 International Symposium of Infectiology and Application, Shanghai, China (Invited speaker)
2019 Australasian Society for Virology, Queenstown, New Zealand.
2019 International Society for Heart and Lung Transplantation, Orlando, USA
2019 TSANZ Annual Scientific Meeting, Sydney, Australia
2018 TSANZ Annual Scientific Meeting, Melbourne, Australia
2017 Australian Respiratory Virology Meeting, Melbourne, Australia (Invited speaker)
2017 Australasian Society for Virology, Adelaide, Australia.
2017 TSANZ Annual Scientific Meeting, Brisbane, Australia
Image credit – ThoughtCo

Lungitude has committed to 3 years of funding a PhD candidate, Steven Hiho, as he continues to work on projects to improve donor- recipient matching.
The last few years has seen the creation of several new computer programs to assess ‘compatibility’ between a lung transplant recipient and a potential donor. These programs use the differences between donor and recipient proteins to give a ‘score’ of compatibility between any particular pair.
Developing better tools for donor- recipient matching may be a key to preventing the development of CLAD, with the added aims of improving and prolonging life post lung transplantation.
Recent Progress
In the first year of his PhD project, Steven has concentrated on looking at blood samples from a group of 310 Alfred lung transplant patients to assess the effectiveness of these different computer programs in their ability to provide a recipient and donor compatibility score, and try and correlate this compatibility/ matching score with the patient outcomes. Put simply, Steven has been trying to work out what level of HLA compatibility is required to give the best post transplant outcomes.
It also appears that not all HLA ‘mismatches’ are equal in their ability to cause rejection. Thus, Steven has looked to see if individual high Risk Epitope Mismatches (REM) that have been shown by other international groups to contribute to development of chronic rejection (CLAD) and reduce survival after lung transplantation, were present in the 310 Alfred patients samples. Whilst there were 27% of HLA high REM in the Alfred patient group, surprisingly these REM did not predictably correlate with CLAD or reduced survival post lung transplant in these patients
In the same 310 patients, Steven also used the computer program HLA Matchmaker to evaluate HLA epitope mismatch load (epMM) to see if he could use this data to predict the immunological risk of developing CLAD and post-transplant survival. This study did show that there was a cut-off level for a specific HLA Class II epMM (≤31) that predicted better patient survival than if the epMM was higher, thus identifying a group of lung transplant recipients with a lower immunological risk of developing CLAD.
These results were significant enough to be submitted as abstracts for potential presentation at the 2020 ISHLT meeting being held in Montreal in April 2020.
Abstracts submitted to 2020 ISHLT Conference:
- Can Avoiding so-called HLA high risk epitope mismatches (REM) improve lung transplant outcomes?
- HLA epitope mismatch load (epMM) allows classification of immunological risk and correlates with patient survival following lung transplantation.
These 2 abstracts were rated highly enough to be accepted for oral presentation at the 2020 ISHLT meeting, however due to COVID 19 the ISHLT meeting was cancelled. Steven is currently in the process of finishing several manuscripts that will report these results and hopefully these will be accepted for publication in a major peer- reviewed transplantation journal.
Expanding on the results of early project work, this year Steven had been working with the VITS/Red Cross tissue typing team to try and work on the best way to use HLA epitopes and epMM scoring clinically, plus better HLA screening of donors, to be able to select more compatible recipients for each potential donor. He is also extending his PhD research to look at other (non-HLA) immunological markers which could also potentially be used in the pre-transplant compatibility assessment of lung donors.
Image – Steven Hiho
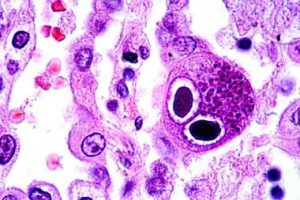
Rejection of the transplanted lung is linked to infection, with one of the most important infections being cytomegalovirus (CMV). Monitoring whether the immune system of a particular lung transplant patient can control the amount of CMV in their blood, would provide information regarding duration of antiviral medication treatment. It is important to define the optimal duration of antiviral medications as these drugs are very expensive, can have significant side effects and can result in resistant strains of CMV too.
Hence there is a requirement for using appropriate immune-system monitoring ‘assays’ to predict the risk of CMV infection in individual transplant recipient. A commercially available test called the QuantiFERON-CMV Monitor assay is currently available and is being used by the Alfred after Lung Transplant was shown to be beneficial (via previous research funded by Lungitude) to test if a patient’s immune system is able to control CMV.
However, this QuantiFERON-CMV Monitor assay only measures responses from one cell type called ‘killer T cells’. A new test called T-Track CMV measures the function of several other important immune cells (effector cells) and claims to be a better predictor of a patient’s ability to fight CMV. In this second project, the research team wanted to directly compare the utility/benefit of QuantiFERON-CMV with the new T-TrackCMV assay to predict the occurrence of CMV infection following lung transplantation.
Recent Progress
T-Track-CMV assays were performed on cryopreserved blood cells from 34 Alfred lung transplant recipients who were transplanted from 2014 to 2015. We compared the results of the T-Track-CMV assay to previously performed QuantiFERON-CMV Monitor assay results from the same recipients. The amount of CMV (CMV virus load) in both the blood and the lung fluid collected post-transplant was compared both assay results.
QuantiFERON-CMV and T-Track-CMV assays were both equally predictive of high level CMV in the blood, with 6% of assay positive recipients showing high blood levels of CMV compared to 94% of assay negative recipients. So both assays were able to show which patients have better immunity to CMV.
However, T-Track-CMV was only slightly better at predicting CMV in the lung fluid than the QuantiFERON-CMV Monitor assay (66% vs 47% respectively), and both assays overall were less predictive of detecting high levels CMV virus levels in lung fluid compared to blood.
Thus, the overall study conclusions are that both commercially available immune monitoring assays appear to predict high level CMV in the blood, however both the T-Track CMV and the QuantiFERON Monitor CVM assays are less beneficial in trying to predict CMV virus levels in the actual transplanted lung.
Abstract Accepted for Conference Presentation:
March 2020: TSANZ Annual Scientific Meeting, Adelaide, Australia
Student thesis completed
JennyLi, Medical Doctor Research Project Monograph: “Assessing cytomegalovirus immunity in lung transplant recipients”
Publications (in preparation):
Jenny Li, Brad Gardiner, Clare Oates, Jie Lin, Sanda Stankovic, Yvonne Cristiano, Bronwyn J. Levvey, Gregory I. Snell, Andrew G. Brooks, Glen P. Westall and Lucy C. Sullivan (submitted to Transplantation May 2020). “Comparison of immune monitoring modalities for assessing cytomegalovirus immunity following lung transplantation”
Image credit – Medscape
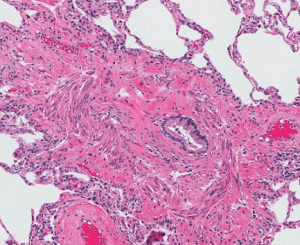
The long term viability of transplanted lungs is ultimately limited by the recipients immune response to the implanted lung graft(s) which in turn leads to the development of CLAD. Apart from evaluating the impact of HLA donor-recipient mismatching and the development of donor specific antibodies (DSA), this project also aims to develop a comprehensive picture about the immune responses after lung transplant to the lung microbiome- this is looking specifically at the vast bacterial, viral and fungal communities that inhabit the lower airways of the lungs. There are also other immune and physiological markers of lung inflammation, rejection and tissue re-modelling that can be detected in blood, lung fluid and lung biopsies. Identifying these early could assist us in preventing long-term damage to the transplanted lungs, plus inform more targeted use of immunosuppression and other treatment therapies and patient-specific regimes.
This project has 6 study aims, with the study specimens (blood /BAL/ tissue biopsies) being sent to the various labs of our collaborators on this important project:
Aim 1: Defining HLA eplet mismatch scores & Immune response in high eplet mismatch Lung transplant recipients (Collaborators: Victorian Transplantation & Immunogenetics Service (VTIS) – linking with Steven Hiho’s PhD project work)
Aim 2:
(a) B-cell specificity, phenotype and function
(Collaborators: Monash University- Prof David Tarlington, Dr Dimmy Zotos)
(b) Antibody-dependant NK cell cytotoxicity
(Collaborators: Melbourne University/Doherty Institute- Dr Lucy Sullivan)
Aim 3: Determine the immunological profiles of the lower airways of lung transplant recipients
Aim 4: Identification of the bacterial, fungal and viral communities in lung transplant recipients
Aim 5: Bioinformatics and integration of microbial, immunological and clinical datasets
(Collaborators: Monash University- Dr Ben Marsland & team)
Aim 6: Applying microarray to describe the molecular phenotype of lung allografts (Collaborator: Alberta Transplant Applied Genome Centre, Alberta, Canada- Prof Philip Halloran)
Recent Progress in 2019 & early 2020
Measurement of Biomarkers II project commenced in April 2018, aiming to initially follow 100 post lung transplant patients for 3 years. Patients enrolled into the study consented to have samples of blood, lung fluid (BAL) and tissue biopsies collected at specific time points when routine post-transplant surveillance bronchoscopies are performed- weeks 2 & 6, and 3,6, 9, 12 & 18 months post transplantation. Specimens are also collected at defined clinical time-points.
Enrolment of 100 lung transplant patients was achieved late 2019, and Alfred Ethics approved an amendment to increase study enrolment to 150. By Feb 2020, a total of 105 patients had been enrolled and more than 50 patients have reached the 12 month time point to date, however the impact of the COVID-19 pandemic resulted in the cessation of new patient enrolment into research projects from 20 March 2020 onwards until further notice.
Due to the high risk posed by undertaking bronchoscopies during the COVID-19 pandemic (eg .potential high risk of virus spread during procedure) all clinical and research bronchoscopies were also ceased, unless absolutely necessary for clinical diagnostic reasons so no research specimens could be collected. Additionally, due to COVID-19, many research labs had shut access to research staff unless they were working specifically on COVID-19 research projects.
Due to the uncertainty regarding how long the COVID-19 pandemic would last, the research teams met (via Zoom) in early May 2020 and decided that the enrolment of new patients into the Biomarkers II Study would cease completely. Thus, only the 105 patients already enrolled in the study could contribute further specimens at the relevant time points, and contribute to the overall analysis.
A significant number of specimens (blood/BAL/Biopsies) from these 105 patients have already been collected and sent to all the participating laboratories & collaborating teams for processing. At present, everyone across the different laboratories in Melbourne is collating all the results and data from specimens already analysed, including the genetic transcript analysis from 4 tissue biopsies sent to the lab in Alberta, Canada prior to the COVID-19 issues. There is still a huge amount of stored specimen processing work for the relevant teams to complete once the laboratories are allowed to re-open.
Hopefully, as the COVID-19 restrictions lift, bronchoscopies on patients already enrolled will be able to continue at important time points (eg 3, 6, 12 & 18 mths post Tx) and additional research specimens and clinical data will be collected for these patients and be able to sent to the various labs again to provide a more complete cohort of data.
There have been quite a few manuscripts by relating to the research supported by Lungitude Foundation accepted for publication past 12 months- these are a listed below along with research abstracts accepted for oral presentation at 2020 International Society of Heart and Lung Transplant (ISHLT) international scientific meeting in Montreal, which was unfortunately cancelled due to COVID-19.
Image credit – Thoracic Key

Lung transplantation (LTx) is an established therapeutic option to improved survival and quality of life for selected patients with advanced severe lung disease. However, the survival after LTx is a median of 6.5 years world-wide, and remains inferior to survival demonstrated in other solid organ transplant groups. Chronic Lung Allograft Dysfunction (CLAD) is regarded as the leading determinant of poor patient survival and the most common manifestation of CLAD is an obstructive phenotype, also commonly known as bronchiolitis obliterans syndrome (BOS) characterised by airflow limitation. There is also another common phenotype of CLAD which is characterised by decreased lung volumes and fibrosis of the lung tissue- this is called restrictive allograft syndrome (RAS) which has a particularly grim prognosis. The identification of predictive factors associated with survival and development of CLAD is imperative to improve outcomes for lung transplant recipients.
The current standard of care utilises spirometry, a routine measurement of lung function, to monitor graft function longitudinally and detect development of rejection after lung transplantation. However, there are significant clinical limitations to spirometric monitoring, as it is relatively insensitive to early and subtle changes in lung function in transplanted lungs. Furthermore, spirometry is not sensitive enough to detect changes in function within the small airways or the alveolar-capillary interface (lung air sacs).
The Forced Oscillometry technique (FOT) is a new ‘simpler’ pulmonary function test that uses ‘normal breathing’ with-out requiring a ‘forced effort’ like spirometry, and uses pressure waves to measure respiratory mechanics and function in the small airways where the injury of CLAD more commonly occurs. FOT testing adds approximately 5 minutes extra to the standard lung function testing process. Studies in different types of lung disease, such as asthma and chronic obstructive lung disease, have shown that FOT can detect lung diseases changes earlier than conventional pulmonary function tests such as spirometry.
Study #1: Researchers will be enrolling any LTx patient who presents for their standard routine post-transplant review where FOT will be done as well as routine lung function spirometry, to be part of a multi-centre cross-sectional cohort study to see if FOT is a better detector of early CLAD versus standard spirometry. They hope to enrol approximately 100 patients from Alfred (and 200+ from St Vincent’s, Sydney) into this study, and ultimately compare the study results with a matched cohort from Toronto, Canada.
Study #2: Researchers will also be measuring FOT in all new lung transplant patients at routine post-transplant reviews – monthly for the first 12 months post LTx, then 3 monthly. Other standard lung function tests including spirometry will be done as per routine follow-up, to provide correlative lung function data and to enable diagnosis of CLAD per international guidelines. They aim to enrol up to 100 Alfred Patients and approximately 100 from St Vincents, Sydney into this study.
Study #3: Additionally, a cohort of patients who are pre-Tx and then get transplanted, will have FOT and a slow vita capacity (SVC ) measurement performed by Dr Kris Nilsen. The SVC test -developed by Dr Nilsen- can measure airway closure [1] and lung stiffness [2] both of which are altered if a LTx patient develops rejection. SVC measurements will be done pre-LTx and at 3 and 12 months post LTx, to provide information about the impact that LTx has on chest wall mechanics, as well as measuring change in lung airway closure and stiffness over the first 12 months after LTx.
[1] Nilsen, K., et al. “Early onset of airway decruitment assessed using the forced oscillation technique in subjects with asthma.” J Appl Physiol (1985) 2019:126(5): 1399-1408.
[2] Nilsen, K., et al. (2018). “Comparison of two methods of determining lung de-recruitment, using the forced
oscillation technique.” Eur J Appl Physiol 118(10): 221302224.X
Recent Progress in 2020 & 2021
Despite the impact of COVID in 2020 and the inability for patients to physically attend the Alfred to have lung function testing, the ‘FOT in Lung Transplantation’ project was able to eventually commence, with the first patient being consented, enrolled and tested at The Alfred in Aug 2020. Brigitte Borg (Head of the Physiology/Lung Function Dept) and Kris Nilsen (Senior scientist- Physiology/Lung Function Dept- funded by Lungitude Foundation 2 days per week) set up a streamlined process within the lung function department to consent and enrol patients into 2 FOT studies. Dr Jai Vazirani (LTx Consultant) and Dr Tom Crowhurst (LTx Fellow) joined the research team and assisted with consenting and enrolment of patients when they attend the transplant clinic for review.
Currently the focus has been on 2 studies being done evaluating the benefit of FOT, and already researchers are obtaining some really important information which has been presented recently at national and international conferences:
Study # 1 update: This is a cross-sectional study where FOT measure measurements are done at one time point, at the same time enrolled study patients have their other routine lung function tests done, to determine if FOT is a better detector of early CLAD versus standard spirometry. This study currently has 57 patients enrolled to date at Alfred and approximately 200 at St Vincent’s Hospital, Sydney. Results from an early analysis in 2 selected cohorts of patients from this study are quite promising, and 2 abstracts submitted to the ISHLT Virtual Meeting (held 24-28 April,2021) were selected to be presented; one as highly selected oral presentation1 and one as a poster presentation2 .
Study # 2 update: This is a longitudinal study which follows patients early from lung transplant and measures FOT along with other routine lung function measures at their first post-transplant lung function test, then monthly during the first 12 months, and 3 monthly thereafter. This study will determine the ability of using FOT testing post LTx to detect early CLAD, and also characterise the different physiological and mechanical changes that occur in the chest wall with LTx. Currently 44 patients at the Alfred have been enrolled and are participating in this longitudinal study, and 37 from St Vincents, Sydney. Enrolments will continue at both hospitals during 2021 and analysis of data will be done as cohorts of the patients reach 12 months post-LTx.
Study # 3 update: This study has only just commenced as there had been a delay in 2020 due to COVID in re- starting the pre-Tx assessment lung function testing. Enrolments for this study are only being done at The Alfred at present.
Presentations on research outcomes related to this project to date:
Oral Presentation @ ISHLT, April 2021- Characterisation of Baseline and Chronic Lung Allograft Dysfunction by Airway Oscillometry: Results of a Multi-centre Cross-Sectional Study. First author and Presenter-David Darley (St Vincent’s, Sydney), J Sim, K Nilsen, R Shirol, B Borg, J Vazirani, B Levvey, G Snell, M Plitt, K Tonga
Poster @ ISHLT, April 2021: Novel measurements of respiratory system mechanics demonstrate important features of donor-recipient lung volume matching that may link to subsequent chronic lung allograft dysfunction. First author and Presenter- Kris Nilsen (Alfred), D Darley, B Levvey, B Borg, J Vazirani, J Sim, R Shirol, M Plitt, G Snell.
Oral presentation at TSANZSRS/ANZSRS Conference, 30 April 21: Transplant donor-recipient lung-volume matching alters novel measurements of respiratory mechanics. First author and Presenter- *Kris Nilsen (Alfred), D Darley, B Levvey, B Borg, J Vazirani, J Sim, R Shirol, M Plitt, G Snell.
* Kris Nilsen won the ANZSRS Robert Jensen Excellence in Respiratory Measurement Prize for the best presentation at the ANZSRS conference in 2021.
Image credit – Tremoflo
Enrichment of cytomegalovirus-induced NKG2C+ Natural Killer cells in the lung allograft.
H1. Harpur, C. M., S. Stankovic, A. Kanagarajah, J. M. L Widjaja, B.J . Levvey, Y. Cristiano, G. I Snell, A. G. Brooks, G. P Westall and L. C. Sullivan (2019). Transplantation. 103:1689-1699.
Consequences of donor-derived passengers (pathogens, cells, biological molecules and proteins) on clinical outcomes
Snell, G.I., S. Hiho, B. J. Levvey, L.C. Sullivan and G.P. Westall (2019). J Heart Lung Transplant. 2019. 38: 902-906
Transfer of donor anti-HLA antibody expression to multiple transplant recipients- a potential variant of the Passenger Lymphocyte Syndrome? Kummrow, M. S. Hiho, F. Hudson, L. Cantwell, W. Mulley, L. D’Orsogna, A. Testro, J. Pavlovic, P. MacDonald, L.C. Sullivan, G. I. Snell and G. P. Westall (2019). Am J Transplant 19(5):1577-1581.
The complex existence of T cells following transplantation: the good, the bad and the simply confusing.
Sullivan, L.C., E. M. Shaw, S. Stankovic, G. I. Snell, A. G. Brooks and G. P. Westall (2019). Invited Review. Clin Transl Immunology 8(9):e1078
T Cells in Transplantation: Friend and Foe.
Sullivan, L. C., E. M. Shaw and G. P. Westall (2018). Transplantation. 102:1970-1971.
Comparison of immune monitoring modalities for assessing cytomegalovirus immunity following lung transplantation.
Jenny Li, Brad Gardiner, Clare Oates, Jie Lin, Sanda Stankovic, Yvonne Cristiano, Bronwyn J. Levvey, Gregory I. Snell, Andrew G. Brooks, Glen P. Westall and Lucy C. Sullivan (submitted to Transplantation May 2020).
Antibody Mediated Rejection: Are We There Yet?
Book Chapter · August 2019 Essentials in Lung Transplantation, pp.79-86DOI: 10.1007/978-3-319- 90933-2_7 GP Westall and L C Sullivan.
Consequences of donor-derived passengers (pathogens, cells, biological molecules and proteins) on clinical outcomes.
J Heart Lung Transplant 38(9) · June 2019 DOI: 10.1016/j.healun.2019.06.019 G Snell, S Hiho, B Levvey, L Sullivan, G Westall.
Enrichment of Cytomegalovirus-induced NKG2C+ Natural Killer Cells in the Lung Allograft. Transplantation. 2019 Aug;103(8):1689-1699 Harpur CM, Stankovic S, Kanagarajah A, Widjaja JML, Levvey BJ, Cristiano Y, Snell GI, Brooks AG, Westall GP, Sullivan LC.
Molecular phenotyping of rejection-related changes in mucosal biopsies from lung transplants.
Am J Transplant. 2020 Apr;20(4):954-966. Halloran K, Parkes MD, Timofte IL, Snell GI, Westall GP, Hachem R, Kreisel D, Levine D, Juvet S, Keshavjee S, Jaksch P, Klepetko W, Hirji A, Weinkauf J, Halloran PF.
Cytomegalovirus replication is associated with enrichment of distinct T cells substes following lung transplantation: A novel therapeutic approach?
LC Sullivan et al Accepted by J Heart Lung Transplant May 2020
$150,000 for the 2019/20 Financial Year

The Jeff Gittus Lung Transplant Fellowship was announced in 2019, funded generously by Liz Gittus and the Gittus Family. The Fellowship provides one annual fellowship grant of up to $10,000 for a research project of 12-18 months total duration, that focuses specifically on improving lung transplant patient outcomes.
For further information please visit our Grants Section.
The inaugural Jeff Gittus Fellowship, funded by the Gittus Family in memory of Jeff, was awarded to Dr Louise Fuller (Physiotherapist) and Ms Christie Emsley (Dietician) for their project – Body Composition and Muscle Morphology after Lung Transplant.

The major cause of reduced long-term survival after lung transplantation is chronic graft rejection—also known as Chronic Lung Allograft Dysfunction (CLAD). Disappointingly, CLAD occurs in ~50% of all lung transplant recipients by 5 years, with long-term results far inferior to other solid-organ transplants.
The introduction of modern medicines called immunosuppressors has improved one form of rejection called cellular rejection, however another form called ‘Antibody Mediated Rejection’ is now believed to be the main cause of organ loss following lung transplantation.
Our immune system is designed to recognise ‘foreign’ invaders such as bacteria and viruses and produce antibodies to fight them off. However, the transplanted lung is also seen as ‘foreign’ to the recipient’s immune system, and antibodies known as donor specific antibodies (DSA) can be produced which attack the transplanted donor lung.
Defeating Transplant Rejection: Antibodies and Strategies to Control Them, aims to identify and understand the production of DSAs and identify the mechanisms that lead to damage of the transplanted lung. Ultimately, researchers anticipate this will lead to new therapies to prevent rejection.
DSAs are routinely measured prior to transplant, and when present they influence whether a lung donor is compatible or not. Researchers also look for the presence of these antibodies after transplantation that may develop in response to the donor lung(s). Their aim is to select the ‘best matched’ donor organ to a particular recipient in order to prevent new antibodies being formed. PhD student, Mr Steven Hiho, who is based at the Australian Red Cross Blood Service has made further inroads to this project.
Recent Progress
In an exciting development during this project, researchers discovered that antibodies that are produced by the donor’s immune cells can also contribute to the immune response in recipients. This area of research has the potential to cause a paradigm shift in the way we think about antibodies that can cause damage to a transplanted lung.
Researchers now have new data showing the donor’s immune cells can persist in the blood of recipients for quite a long time following transplantation. However, they have found that the number and type of the donor’s immune cells is extremely variable between different recipients. Work is underway to determine the correlation between these findings and the later development of donor-specific antibodies.
In new research, work is being done to establish the link between viral infection and antibody-mediated rejection. In particular, researchers are investigating possible links between infection with a virus called cytomegalovirus (CMV) and antibody- mediated rejection.
In Australia, 50% of individuals are infected with cytomegalovirus (CMV), even so, the virus persists without symptoms in healthy people. However uncontrolled CMV infections can occur in states of immunosuppression, such as following lung transplantation, with approximately 50% of recipients experiencing active viral infections.
Researchers have previously observed that CMV replication was associated with the development of CLAD, however the link between the two is not well understood. Therefore, their new work is aimed at finding novel therapeutics and diagnostics to prevent CMV infections, thereby also limiting antibody-mediated rejection.
The researchers are generating fantastic data on this project, which has resulted in a recent patent application. Moreover, they have received seed funding from the University of Melbourne to continue work in this area (University of Melbourne School of Biomedical Sciences Translational Research Award).
The research team are very pleased with the considerable progress they have made into this project and anticipate further progression as they continue in the coming year.
Image credit – ThoughtCo

Lungitude has committed to 3 years of funding a PhD candidate, Steven Hiho, as he continues to work on projects to improve donor- recipient matching.
The last few years has seen the creation of several new computer programs to assess ‘compatibility’ between a lung transplant recipient and a potential donor. These programs use the differences between donor and recipient proteins to give a ‘score’ of compatibility between any particular pair.
Developing better tools for donor- recipient matching may be a key to preventing the development of CLAD, with the added aims of improving and prolonging life post lung transplantation.
Recent Progress
In the first year of his PhD project, Steven has concentrated on looking at blood samples from a group of 310 Alfred lung transplant patients to assess the effectiveness of these different computer programs in their ability to provide a recipient and donor compatibility score, and try and correlate this compatibility/ matching score with the patient outcomes. Put simply, Steven has been trying to work out what level of HLA compatibility is required to give the best post transplant outcomes.
It also appears that not all HLA ‘mismatches’ are equal in their ability to cause rejection. Thus, Steven has looked to see if individual high Risk Epitope Mismatches (REM) that have been shown by other international groups to contribute to development of chronic rejection (CLAD) and reduce survival after lung transplantation, were present in the 310 Alfred patients samples. Whilst there were 27% of HLA high REM in the Alfred patient group, surprisingly these REM did not predictably correlate with CLAD or reduced survival post lung transplant in these patients
In the same 310 patients, Steven also used the computer program HLA Matchmaker to evaluate HLA epitope mismatch load (epMM) to see if he could use this data to predict the immunological risk of developing CLAD and post-transplant survival. This study did show that there was a cut-off level for a specific HLA Class II epMM (≤31) that predicted better patient survival than if the epMM was higher, thus identifying a group of lung transplant recipients with a lower immunological risk of developing CLAD.
Expanding on the results of early project work, this year Steven had been working with the VITS/Red Cross tissue typing team to try and work on the best way to use HLA epitopes and epMM scoring clinically, plus better HLA screening of donors, to be able to select more compatible recipients for each potential donor. He is also extending his PhD research to look at other (non-HLA) immunological markers which could also potentially be used in the pre-transplant compatibility assessment of lung donors.
Image – Steven Hiho

This project will continue to extend on previously funded CMV research work, further evaluating the link between viruses and rejection.
Rejection of the transplanted lung is linked to infection, with the most important being Cytomegalovirus (CMV). Monitoring whether the immune system of a particular lung transplant patient can control the amount of CMV in their blood, will provide information regarding duration of antiviral medication treatment.
A commercially available test called the QuantiFERON-CMV assay is currently available and is being used by The Alfred (via previous research funded by Lungitude) to test if a patient’s immune system is able to control CMV. A new test called T-Track CMV measures the function of several other important immune cells and claims to be a better predictor of a patient’s ability to fight CMV. In this second project, researchers aim to compare the utility/benefit of QuantiFERON-CMV with the new T-Track CMV assay.
Recent Progress
T-Track-CMV assays were performed on cryopreserved blood cells from 34 Alfred lung transplant recipients. We compared the results of the T-Track- CMV assay to previously performed QuantiFERON-CMV monitor assay results from the same recipients. The amount of CMV virus load in both the blood and the lung fluid collected post-transplant was compared in both assay results.
QuantiFERON-CMV and T-Track-CMV assays were both equally predictive of high level CMV in the blood. Both were able to show which patients have better immunity to CMV. However, T-Track- CMV was only slightly better at predicting CMV in the lung fluid than the QuantiFERON- CMV Monitor assay, and both assays overall were less predictive of detecting high levels CMV virus levels in lung fluid compared to blood.
Thus, the overall study conclusions are that both commercially available immune monitoring assays appear to predict high level CMV in the blood, however both the T-Track CMV and the QuantiFERON Monitor CVM assays are less beneficial in trying to predict CMV virus levels in the actual transplanted lung.
Image credit – Medscape

This project extends the previous Biomarker I study — Identifying of biomarkers of immune function and infection in the blood and lung aiming to predict episodes of acute rejection and the onset of chronic lung allograft dysfunction (CLAD) following lung transplantion. Identifying immune and physiological markers early could assist in preventing long-term damage to the transplanted lungs, plus inform more targeted use of immunosuppression and other treatment therapies and patient-specific regimes.
Recent Progress
The Measurement of Biomarkers II project aimed to initially follow 100 post lung transplant patients for 3 years. Patients enrolled into the study consented to have samples of blood, lung fluid (BAL) and tissue biopsies collected at specific time points.
Enrolment of 100 lung transplant patients was achieved and Alfred Ethics approved an amendment to increase study enrolment to 150.
As at Feb 2020, a total of 105 patients had been enrolled and more than 50 patients had reached the 12 month time point to date, however the impact and uncertainty due to COVID-19 resulted in the cessation of new patient enrolment into research projects from 20 March 2020 onwards until further notice.
Due to the high risk posed by undertaking bronchoscopies during COVID-19 all clinical and research bronchoscopies were also ceased, unless absolutely necessary for clinical diagnostic reasons so no research specimens could be collected.
Additionally, due to COVID-19, many research labs had shut access to research staff unless they were working specifically on COVID-19 research projects.
Thus, only the 105 patients already enrolled in the study could contribute further specimens at the relevant time points, and contribute to the overall analysis.
A significant number of specimens (blood/BAL/Biopsies) from these 105 patients have already been collected and sent to all the participating laboratories & collaborating teams for processing. At present, everyone across the different laboratories in Melbourne is collating all the results and data from specimens already analysed.
There is still a huge amount of stored specimen processing work for the relevant teams to complete once the laboratories are allowed to re-open. Hopefully, as the COVID-19 restrictions lift, bronchoscopies on patients already enrolled will be able to continue at important time points and additional research specimens and clinical data will be collected for these patients and be able to sent to the various labs again to provide a more complete cohort of data.
There have been quite a few manuscripts relating to the research supported by Lungitude Foundation accepted for publication past 12 months (listed on pg 6) along with research abstracts accepted for oral presentation at 2020 International Society of Heart and Lung Transplant (ISHLT) international scientific meeting in Montreal, which was unfortunately cancelled due to COVID-19.
Image credit – Thoracic Key
Enrichment of cytomegalovirus-induced NKG2C+ Natural Killer cells in the lung allograft. H1. Harpur, C. M., S. Stankovic, A. Kanagarajah, J. M. L Widjaja, B.J . Levvey, Y. Cristiano, G. I Snell, A. G. Brooks, G. P Westall and L. C. Sullivan (2019). Transplantation. 103:1689-1699.
Consequences of donor-derived passengers (pathogens, cells, biological molecules and proteins) on clinical outcomes Snell, G.I., S. Hiho, B. J. Levvey, L.C. Sullivan and G.P. Westall (2019). J Heart Lung Transplant. 2019. 38: 902-906
Transfer of donor anti-HLA antibody expression to multiple transplant recipients- a potential variant of the Passenger Lymphocyte Syndrome? Kummrow, M. S. Hiho, F. Hudson, L. Cantwell, W. Mulley, L. D’Orsogna, A. Testro, J. Pavlovic, P. MacDonald, L.C. Sullivan, G. I. Snell and G. P. Westall (2019). Am J Transplant 19(5):1577-1581.
The complex existence of T cells following transplantation: the good, the bad and the simply confusing. Sullivan, L.C., E. M. Shaw, S. Stankovic, G. I. Snell, A. G. Brooks and G. P. Westall (2019). Invited Review. Clin Transl Immunology 8(9):e1078
T Cells in Transplantation: Friend and Foe Sullivan, L. C., E. M. Shaw and G. P. Westall (2018). Transplantation. 102:1970-1971.
Comparison of immune monitoring modalities for assessing cytomegalovirus immunity following lung transplantation. Jenny Li, Brad Gardiner, Clare Oates, Jie Lin, Sanda Stankovic, Yvonne Cristiano, Bronwyn J. Levvey, Gregory I. Snell, Andrew G. Brooks, Glen P. Westall and Lucy C. Sullivan (submitted to Transplantation May 2020).
Antibody Mediated Rejection: Are We There Yet? Book Chapter · August 2019 Essentials in Lung Transplantation, pp.79-86DOI: 10.1007/978-3-319- 90933-2_7 GP Westall and L C Sullivan.
Consequences of donor-derived passengers (pathogens, cells, biological molecules and proteins) on clinical outcomes. J Heart Lung Transplant 38(9) · June 2019 DOI: 10.1016/j.healun.2019.06.019 G Snell, S Hiho, B Levvey, L Sullivan, G Westall.
Enrichment of Cytomegalovirus-induced NKG2C+ Natural Killer Cells in the Lung Allograft. Transplantation. 2019 Aug;103(8):1689-1699 Harpur CM, Stankovic S, Kanagarajah A, Widjaja JML, Levvey BJ, Cristiano Y, Snell GI, Brooks AG, Westall GP, Sullivan LC.
Molecular phenotyping of rejection-related changes in mucosal biopsies from lung transplants. Am J Transplant. 2020 Apr;20(4):954-966. Halloran K, Parkes MD, Timofte IL, Snell GI, Westall GP, Hachem R, Kreisel D, Levine D, Juvet S, Keshavjee S, Jaksch P, Klepetko W, Hirji A, Weinkauf J, Halloran PF.
Cytomegalovirus replication is associated with enrichment of distinct T cells substes following lung transplantation: A novel therapeutic approach? LC Sullivan et al Accepted by J Heart Lung Transplant May 2020

Lungitude, with part support from a generous donor, funded a QuantStudio 5 real-time PCR machine that will be used for lung transplant antibody applications and other research projects.
The machine will reside at the Peter Doherty Institute, where Dr Lucy Sullivan conducts her research.
We were pleased to hear that this machine was also temporarily loaned to undertake important COVID-19 research.
$115,000 for the 2018/9 Financial Year


The major cause of reduced long-term survival after lung transplantation is chronic graft rejection – also known as Chronic Lung Allograft Dysfunction (CLAD) – that disappointingly occurs in ~50% of all lung transplant recipients by 5 years. Antibodies that attack transplanted lung tissue, known as donor specific antibodies (DSA) are a suspected cause of CLAD (called antibody-mediated rejection). Our immune system is designed to recognise “foreign” invaders such as bacteria and viruses and produce antibodies to them. However, the transplanted lung is seen as “foreign” to the recipient’s immune system, causing DSA to be produced which attack the donor lung. Defeating Transplant Rejection: Antibodies and Strategies to Control Them, aims to identify and understand the mechanisms of antibody production that mediate destruction for the purpose of ultimately identifying targeted therapies to prevent rejection.
DSAs are routinely measured prior to transplant to try to avoid transplantation of a lung where the recipient has existing DSA to that particular organ, where possible. DSAs are also measured after transplantation to determine if new DSAs are being produced. The key is selecting the “best matched” donor organ in order to prevent new DSAs being produced. Researchers have continued to make inroads to this project, with 3 students (2 PhD and 1 medical student) joining to work on this research. One of these PhD students, Mr Steven Hiho, is based at the Australian Red Cross Blood Service and has already been a co-author on a recent publication on this project (see below). In this manuscript, researchers showed that “eplet” mismatches between the donor and recipient in a protein called HLA-DQ can lead to the production of DSA. Mr Hiho’s future work is aimed at identifying the “high-risk” mismatches that cause trouble for the recipient so that the team can avoid these mismatches in future transplants. This process is underway but has been delayed by a change in the definition of CLAD according to international guidelines. This therefore requires the researchers’ entire cohort in this study (~400 recipients) to be re-analyzed by this new definition of CLAD. Researchers have started this process and anticipate completion around mid 2019.
Another aspect of Mr Hiho’s project is to compare old donor/recipient matching techniques with newer, more sensitive methods and relate this to lung transplant outcomes. It is hoped that this research will lead to better methods of donor selection being adopted on a national level (see other project below).
In 2018, a medical student, Ms Catia Fernandes Monteiro, completed a pilot project aimed at identifying the specific immune cells that cause damage to the lung following the production of DSA. The research on this project was extremely productive and successful, ascertaining that a group of white blood cells called “natural killer” or NK cells have heightened activity in recipients with DSA. This research has been presented at a conference and several seminars and written for publication in 2019.
In an exciting new development in this project researchers now believe that the antibodies that are produced by the donor’s immune cells can also contribute to antibodies present in recipients. These novel findings were recently accepted for publication in the top-ranked transplantation journal, American Journal of Transplantation. Researchers will continue to pursue this area of research as it has the potential to cause a paradigm shift in the way we think about antibodies that can cause damage to a transplanted lung.
Publications:
“HLA class II Eplet mismatch predicts De Novo DSA formation post lung transplant”.
Walton DC, Cantwell L, Hiho S, Ta J, Wright S, Sullivan LC, Snell GI, Westall GP. Transplant Immunology 2018 51:73- 75.
“Transfer of donor anti-HLA antibody expression to multiple transplant recipients: A potential variant of the passenger lymphocyte syndrome?” Kummrow M, Hiho S, Hudson F, Cantwell L, Mulley WR, D’Orsogna L, Testro A, Pavlovic J, MacDonald P, Sullivan LC, Snell GI, Westall GP. Am J Transplant. 2019 Jan 17.
Image credit – ThoughtCo

The last few years has seen the creation of several new computer programs to assess “compatibility” between a lung transplant recipient and a potential donor. These programs use differences between donor and recipient proteins and give a “score” of compatibility between any particular pair. This PhD project will compare the effectiveness of these different computer programs in their ability to predict which patients will have less rejection. Initially, researchers will look at patients that have been previously transplanted and assign them ‘compatibility scores’ with each computer program and compare these scores with rejection. By doing so, researchers can guide the Red Cross on which computer program they should use to give a “score” between a recipient and a potential donor.
In addition, researchers predict that not all protein “mismatches” are equal in their ability to cause rejection. Researchers plan to interrogate each computer program to identify individual “taboo” protein mismatches that are seen repeatedly in patients that have rejection, rather than to use the overall “score” generated by the program.
When researchers identify such “taboo” mismatches they will undertake a laboratory assessment to determine how the body reacts to these proteins to result in rejection of the transplanted lungs. This project fits extremely well into The Alfred’s key goals of seeking a better understanding of what might cause CLAD, and developing better tools for donor- recipient matching may be key to preventing the development of CLAD, with the added aims of improving and prolonging life post lung transplantation.
Image – Steven Hiho

Rejection of the transplanted lung is linked to infection, with the most important being cytomegalovirus (CMV). Monitoring whether the immune system of a particular lung transplant patient can control the amount of CMV in their blood, would provide information regarding duration of antiviral medication treatment. It is important to define the optimal duration of antiviral medications as these drugs are very expensive, can have significant side effects and can result in resistant strains of CMV too. Hence there is a requirement for using appropriate immune-system monitoring ‘assays’ to predict the risk of CMV infection in individual transplant recipient. A commercially available test called the QuantiFERON-CMV assay is currently available and is being used by the Alfred after Lung Tx was shown to be beneficial (via previous research funded by Lungitude) to test if a patient’s immune system is able to control CMV.
However, this QuantiFERON-CMV monitoring assay only measures responses from one cell type called ‘killer T cells’. A new test called T-Track CMV measures the function of several other important immune cells (effector cells) and claims to be a better predictor of a patient’s ability to fight CMV. In this second project, researchers aim to directly compare the utility/benefit of QuantiFERON-CMV with the new T-Track CMV assay to predict the occurrence of CMV infection following lung transplantation.
Researchers will use stored blood collected from 40 post-transplant patients who have already been enrolled into the previous Biomarkers I project and use these samples to run the T-Track CMV assay. Researchers will then compare the T-Track CMV results with the same patients QuantiFERON-CMV test results to see if the new test is more predictive of CMV infection or immunity.
Image credit – Medscape

This project extends the previous Biomarker I study- Identifying of biomarkers of immune function and infection in the blood and lung aiming to predict episodes of acute rejection and the onset of chronic lung allograft dysfunction (CLAD) following LTx. This project, which commenced in April 2018, will follow a cohort of 100 post lung transplant patients for 3 years with specimens collected at all bronchoscopies and also at defined clinical time-points.
An additional Research Nurse/Coordinator will be funded to facilitate collection and recording of data for of all the bronchoscopic (BAL) and blood specimens taken at each bronchoscopy. Funding will also employ a Research Lab Assistant in the Monash laboratory to process the specimens being collected for this project as well as the other 2 projects outlined previously.
Image credit – Thoracic Key

Funding of a dedicated Lung Transplant Research Laptop/ software and SPSS Statistical Software:
- Computers, software or any other forms of IT for use in clinical research need to be funded via research grants or staff have to use their own personal computers and personally purchase and pay the cost for statistical software.
- Alfred staff are not permitted to install statistical software packages and other research programs on hospital computers.
- The laptop computer will be kept in the Lung Transplant Department, and will be available for use by various members of the Lung Tx research team for all projects supported by Lungitude and other clinical research, under the supervision of Prof Greg Snell & A/Prof Bronwyn Levvey
- Research Staff will be able to use the computer to record and collate research data, undertake statistical analysis of the various research projects underway, and also for preparing manuscripts and power-point presentations for conferences and meetings.
- The computer also has the capability for use in research webinars and teleconferences, facilities that are not widely available in the Respiratory Medicine Dept. and limited also around the hospital.
Image credit – SPSS

Lungitude also facilitated patient funding for the Transplant Gym including the donation of a state of the art bike in memory of Tash Tripp.
Image – Tash Tripp’s family and supporters
$90,000 for the 2017/8 Financial Year
Each of us (except identical twins) has a different set of HLA proteins that sit on the surface of the cells within the lung (and other organs). This genetic signature differs between transplant recipients and their donor lung and is what drives the immune system of some transplant recipients to “reject” their lung. Historically, there have been relatively crude tools to assess the compatibility of the HLA of the donor with that of the recipient. In collaboration with the Red Cross, The Alfred’s Lung Transplant Service Team are exploring how the HLA Matchmaker program may allow them in the future to better match donor lungs with the “best fit” recipient. By improving donor/recipient matching, they will reduce the likelihood of subsequent rejection thereby increasing survival following lung transplantation.
The HLA Matchmaker program allows the team to determine HLA proteins at a molecular level. Through a greater understanding of the precise shape and size of the HLA molecules, they can better predict which HLA proteins are more likely to switch on the transplant recipient’s immune system and trigger an episode of rejection. In recent work that they published in the prestigious “American Journal of Transplantation” they used the HLA Matchmaker program to show that lung transplant recipients whose HLA proteins were structurally very different to those of their donor were more likely to develop chronic rejection. In collaboration with the labs of Professor David Tarlinton (Monash University) and Professor Andrew Brookes (University of Melbourne) they will now explore how these structural differences in the HLA molecule switch on the immune system.
The success of this program will be two-fold. Through better matching of donors and recipients they will improve donor organ allocation, but additionally through a greater understanding of the immune pathways leading to rejection they can better target their anti-rejection therapies.
Image credit – bethematch.org
The Alfred Lung Transplant Service’s ‘Molecular Microscope’ Diagnostic System collaboration (the INTERLUNG Study) with the University of Alberta investigators and other international lung transplant units is now well underway. The Study collects small pieces of the transplanted lung in people with a small drop in lung performance, from both the sponge of the lung and the airways leading to it, and analyses them in the standard way under an ordinary microscope, comparing these to genetic analyses in Canada. The standard approach is often non-specific and does not help to guide treatment. The new genetic approach appears to be giving different answers that suggest alternative treatments – potentially helping lungs at a time before lung function is lost permanently.
Historically, small pieces of the sponge of the lung are obtained to check for rejection, but the INTERLUNG Study is suggesting the potential of getting similar, or even better, results f rom bits of the lung airways. This would be an important innovation – noting airway tissue is easier and safer to obtain.
The INTERLUNG Study is ongoing, as the team now tries to link the genetic results with long-term lung performance and different types of rejection. The work will be presented at next year’s International Society of Lung Transplant Meeting and a portion will be submitted for publication soon.
The Lungitude Foundation also supports the legacy of The Margaret Pratt Heart Lung Transplant Research Foundation which has funded the following projects:
$180,000 over 2 years (2017 & 2018)
- A study of QuantiFERON-CMV-Directed CMV prophylaxis versus Standard Of Care to reduce late Cytomegalovirus (CMV) reactivation in patients undergoing lung transplantation.
- A monitoring study using QuantiFERON to measure ‘net’ immunosuppression post lung transplantation.
- A phase 2 randomised, placebo controlled trial of bone-marrow derived mesenchymal stromal Cell infusions as treatment for new on-set chronic lung allograft dysfunction.
- Identifying non-invasive biomarkers of acute and chronic lung allograft dysfunction following lung transplantation.
$120,000* over 4 years (2017-2021)
Match funded by The Alfred Foundation’s supporters – Total of $240,000
- Defeating Lung Transplant Rejection: Antibodies and Strategies to Reject Them

Lung transplant research at The Alfred continues to flourish. The Margaret Pratt Foundation has supported the research team in the running of several projects, and The Alfred’s Lung Transplant Research Team we have been excited by the progress of the research. Researcher Yvonne Cristiano has provided an update on these projects below.
- A study of QuantiFERON-CMV-Directed CMV prophylaxis versus Standard Of Care to reduce late Cytomegalovirus (CMV) reactivation in patients undergoing lung transplantation.
It was an exciting moment this year when our final participant completed this study in April. Since then the data has been analysed and we are thrilled to find that the primary aim of the project was met. The finding that QuantiFERON-CMV-Directed Care could reduce the incidence of CMV reactivation in the transplanted lung has led to a change in our clinical practice. QuantiFERON-CMV monitoring has now been introduced into our protocols for the care of our patients post lung transplant and our CMV management. This project was conducted over 5years involving 118 participants, and now with the introduction of this new clinical test, we hope to see this correlating to a reduction in CMV reactivation and the side effects CMV has on lung transplant recipients.
- A monitoring study using QuantiFERON to measure ‘net’ immunosuppression post lung transplantation.
This project recruited its 80th and final patient in July this year, with participant completion scheduled for July 2018. The QuantiFERON-Monitor blood tests measures the overall strength of a person immune system, which we are collecting at several time point during the first year after transplant. This project aims to show us the potential of this blood test to provide extra information regarding an individual’s immune system, and the possibility for guiding anti-rejection medications after transplantation.
- A phase 2 randomised, placebo controlled trial of bone-marrow derived mesenchymal stromal Cell infusions as treatment for new on-set chronic lung allograft dysfunction.
Managing chronic rejection after lung transplantation continues to be a challenge for all Lung Transplant teams. This projects is being led by Prince Charles Hospital in Queensland. The Alfred has recently obtained Ethics Approval to be a part of this project, and we have had our first patient undergo this therapy. The idea of using mesenchymal stromal cells to decrease the immune system and stop the progression of rejection is a novel therapy, with great potential to assist in the long term survival of lung transplant recipients. This project will continue over the next 5 years, with it anticipated to complete in 2022.
- Identifying non-invasive biomarkers of acute and chronic lung allograft dysfunction following lung transplantation.
Throughout this project many non-invasive biomarkers have been examined, some of these include Ativan, Follistatin, Natural Killer Cells, B Cells, De novo DSA and Cytokines of Aspergillus. There are 137 patients taking part in this project, with the final patient due to complete their participation in January 2018. This project has successfully answered many questions and in doing so raised many more. Analysis of the data from this project remains ongoing, along with analysis of the specimens. As a result of this project, we in the process of development a subsequent project (Biomarker 2), which aims to investigate the biomarkers influencing chronic lung allograft dysfunction (CLAD) further.

The major cause of reduced long-term survival after lung transplantation is chronic graft rejection – also known as Chronic Lung Allograft Dysfunction (CLAD) – that disappointingly occurs to some degree in 49% of all lung transplant recipients by 5 years. It is dif cult to predict which patients will develop CLAD, which occurs despite these patients being treated with anti-rejection drugs life-long.
Antibodies that attack the donor lung (antibody-mediated rejection – AMR) have been suspected as an important potential cause of lung failure and CLAD post lung transplantation. Our immune system naturally forms antibodies as a protective response against bacteria and viruses. In the context of transplantation, antibodies are good when they are ready to attack foreign invaders that can lead to illness, but antibodies can also be ready to attack foreign tissue – such as the donor lung.
Currently, antibodies that can attack transplanted lung tissue, known as donor speci c antibodies (DSA), can be measured prior to transplantation with the aim of selecting a donor organ that minimises the risk of rejection. DSA levels can also be measured post transplantation to see if the patient’s immune system is actually causing harm to the patient’s new lungs. Identi cation and de nition of speci c types of DSA and their incidence in patients undergoing lung transplantation is key to understanding how the immune system makes such antibodies and how they contribute to AMR and ultimately to chronic rejection.
The team from The Alfred Lung Transplant Service are collaborating with the Red Cross / Victorian Transplant Immunology Service to be able to more ef ciently detect and measure the presence of these graft-damaging antibodies in lung transplant patient’s blood samples. This collaboration also aims to develop a computerised matching program that will accurately identify problematic antibodies pre- transplant that will enable a better match between a donor and potential lung recipient, reducing the risk of antibodies causing the development of CLAD after transplant.
The funding provided by this grant has thus far enabled the recruitment of a senior post-doctorate researcher, Dr Lucy Sullivan, to oversee the conduct of this very important project. So far the computer matching program has been used to demonstrate in a sub-set of lung transplant patients that better matching of donor to recipient protects against CLAD. This research was published in the prestigious journal, American Journal of Transplantation. Additionally, in a small group of recipients the team have shown that better matching also reduces the appearance of these antibodies.
This research was presented at the International Society of Heart and Lung Transplant conference in San Francisco in April 2017.
In collaboration with researchers at Monash University, the team have also started a project to identify the specific immune cells that produce these antibodies. So far, they have shown that the number of “B cells” is significantly increased in lung transplant patients that also have damaging antibodies. They are now investigating the characteristics and appearance of these “B cells”. Furthermore, in collaboration with Doherty Institute they are using advanced laboratory techniques to identify other specific immune cells that cause damage to the lung following the production of antibodies. They believe these cells to be a group of white blood cells called “natural killer” or NK cells. So far, they have identified that certain groups of NK cells will kill other cells when antibodies are present.
The results, to date, from all aspects of this project are very pleasing, and the team anticipate being able to make considerable progress as they continue into 2018-19. Already this study is contributing greatly to their understanding of how and why harmful antibodies are produced and how to potentially avoid this process occurring after lung transplantation.
Publication:
HLA Matching at the Eplet Level Protects Against Chronic Lung Allograft Dysfunction.
Walton DC, Hiho SJ, Cantwell LS, Diviney MB, Wright ST, Snell GI, Paraskeva MA, Westall GP.
Am J Transplant. 2016 Sep;16(9):2695-703. doi: 10.1111/ajt.13798. Epub 2016 Apr 21
Learn more about Advocacy.